Fluorescence-Guided Surgery: Enlightened Approach to an Ancient Cancer Treatment
Surgery is an ancient medical disclipline, with the first well-documented operations dating back at least 3000 years. Until the Industrial Revolution of the late 18th century, however, surgeons were incapable of overcoming the three main obstacles that had plagued the profession since its inception: pain, bleeding, and infection. The introduction of painkillers, antibiotics, and sophisticated surgical techniques would eventually transform surgery from a risky “art” into a medical discipline capable of treating many diseases.
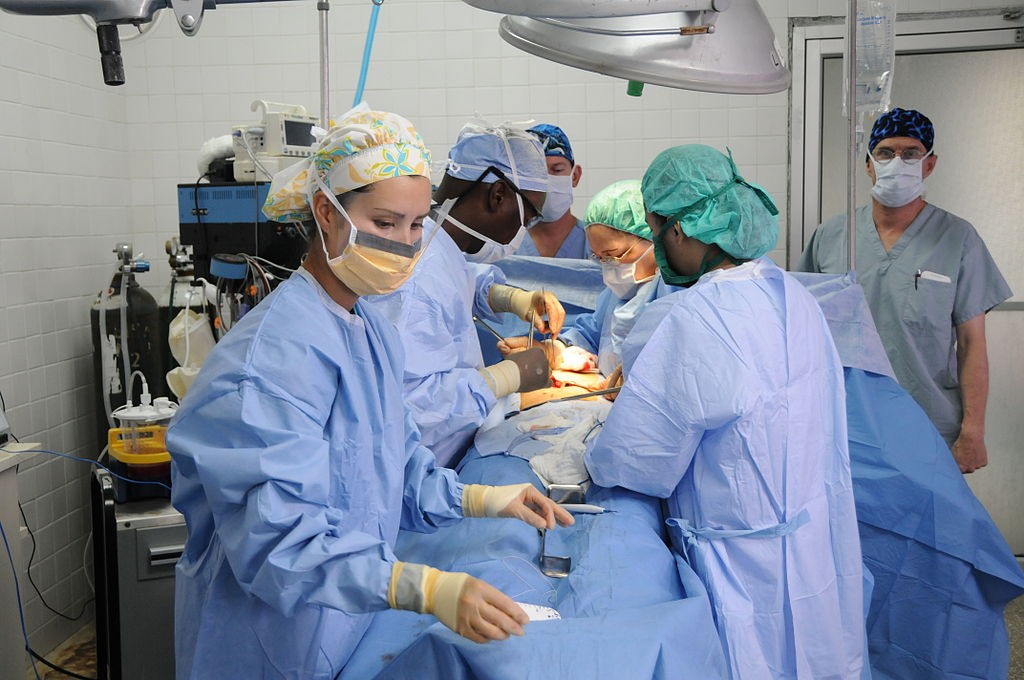
When it comes to the field of oncology, however, surgery’s progress has been hampered by the fact that surgeons must rely primarily on vision and touch (palpation) to gather information about the precise location of a cancerous growth. Certainly the surgeon’s eyes and hands are useful instruments for detecting anatomical structures, but they are incapable of detecting stray cancer cells.
Moreover, the detection of cancer by human inspection and palpation is a highly subjective process, and is often insufficient for distinguishing healthy tissues from malignant lesions. These limitations often result in either incomplete surgery or unnecessary removal of healthy tissue, or both. In most situations, when a surgeon attempts to cut out a tumor, it is unclear as to whether some cancer still remains in the periphery of the surgical wound site, the so-called margin.
Such residual cancer can spawn recurrences down the road, often resulting in more aggressive disease and forcing the oncologist to respond with more aggressive treatments such as high-dose chemotherapy. Fortunately, modern science is constantly finding new ways to improve the use of old tools, and this has certainly been the case with cancer surgery.
The Advent of Flourescence-Guided Surgery
After World War II, medical doctors began using microscopes and various magnifying lenses in the operating room in order to zoom in on the tumor tissue or on minute patches of cancer. At the same time, a fluorescent dye called fluorescein came into use for diagnostic purposes, and other contrast agents were subsequently developed and used during surgery.
Before going further, it may be helpful to explain what is meant by fluorescence—the “glowing” that is seen with fluorescein and other contrast agents. This phenomenon occurs when a molecule absorbs a photon of light. The molecule is activated at a certain wavelength of light, which then triggers the release of photons at a longer wavelength. These photon emissions can be sensed with a highly sensitive camera. The camera can inspect the entire operating field in real time, with color and fluorescence imaging. The interpretation of these images is primarily based on the amount of fluorescence detected.
One of the first fluorescing agents was 5-aminolevulinic acid (5-ALA). This agent tends to concentrate in malignant tissues and helps generate a photosensitizer called protoporphyrin IX (PpIX), a naturally fluorescing substance. The use of 5-ALA in photodynamic diagnosis has shown great promise as a way to maximize the extent of surgical removal of brain tumors (gliomas and meningiomas) while minimizing damage to normal brain tissues. Brain tumor tissues produced a red-pink fluorescence, with diagnostic accuracies of up to 90% for distinguishing meningiomas from normal brain tissue, as reported in the March 2014 issue of Neurosurgery.
In a broad assessment of recent developments in surgical oncology, scientists from Groningen (Netherlands) and Birmingham, Alabama (USA) concluded that, over the past decade, there has been a major trend towards fluorescence imaging in cancer surgery. In addition, they contend that
Advantages of Fluorescence-Guided Surgery
As mentioned earlier, a major advantage of FGS is the ability to address cancer that would otherwise be hidden from the surgeon during the operation. Cancer surgeons are striving for “clean” or negative margins, meaning no cancer can be found in the area surrounding the surgical site. Obtaining such “clean” margins is a major strategy for reducing the risk of tumor recurrences.
Many breast tumors, for example, cannot be felt by touch (palpation), and positive margin rates at the time of surgery can be as high as 49%. In some cases, ultrasound or x-ray fluoroscopic imaging have been tried during surgery, but these modalities lack the ability to specifically detect cancer cells, and moreover they expose the patient and caregiver to ionizing radiation, or require direct contact with the body. MRI and CT scanning have played a role as well, but these systems are costly and complex.
By comparison, fluorescence-based imaging is more cost-effective and easier to implement during surgery. Additionally, the light-based approach does not entail any exposure to ionizing radiation, making it an inherently safe technique as long as attention is paid to the level of laser illumination. Another advantage of FGS is its efficiency.
In the typical clinical setting, the surgeon obtains a tissue sample, which is sent to the pathologist for analysis. The standard pathology report typically takes 5–7 days to obtain, and meanwhile the patient is recovering from the operation. If the report indicates the presence of cancer in the surgical margins, it is often neither ideal nor practical to go back in surgically to remove the tissue. A far more preferable strategy is to provide the surgeon with rapid or direct feedback during the operation, so that adjustments can be made in the procedure and overall treatment strategy in order to improve the outcome. This is precisely what occurs in the context of the fluorescence-guided approach. When the surgeon is empowered to see areas affected by cancer, he or she then has the option of either removing the tissue surgically or using photodynamic therapy (PDT) to eliminate the left-over cancer.
The ideal situation may be to use a photosensitizer that aids in detecting the cancer, remove the bulk of the disease surgically, then shine light of a specific wavelength in the surgical margins to activate the photosensitizer, thus triggering the photodynamic destruction of any residual cancer. This combining of diagnostics and therapy is now referred to as Theranostics, and its elegant practicality could presage the medicine of the future.
Going Deeper with Targeted Fluorescent Probes
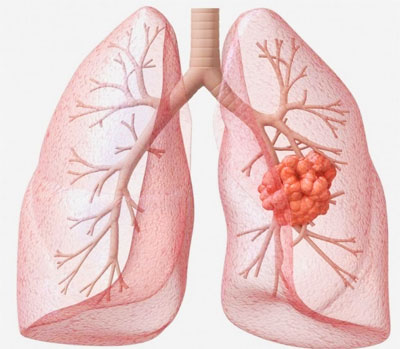
The extent to which fluorescence-based optical imaging in the clinic can enhance the surgeon’s vision depends to a large extent on the site of application. Tumors located near the surface of the skin are easily visualized. Classic examples include skin tumors, bladder tumors, head and neck cancer, melanoma, and peritoneal metastases resulting from ovarian cancer and colorectal cancer. On the other hand, deeper tumors such as sarcomas and breast tumors pose a greater challenge due to the overlay of fat, muscle or glandular tissue. The penetration depth of visible light is generally quite limited, but it can reach up to two centimeters when wavelengths in the near infrared (NIR) part of the light spectrum are used.
According to a report out of Leiden University Medical Center in Leiden (The Netherlands), optical imaging that exploits invisible NIR fluorescent light has the potential to bolster cancer surgery outcomes, minimize the time patients are under anaesthesia, and reduce health-care costs. This is largely due to NIR’s improved contrast and depth of tissue penetration when compared to visible light, as reported in the September 2013 issue of Nature Reviews: Clinical Oncology.
Another way to improve the detection of cancer is through the use of those “guided missiles” called antibodies. These proteins are generated naturally by the human immune system. They can also be synthesized in the laboratory to specifically bind to certain parts of tumor cells, such as the tumor’s growth signalling receptors. Numerous studies have shown that targeting a tumor-specific receptor with fluorescence-labelled antibodies can improve the visualization of cancer in real time during surgery, either using visible light or the NIR part of the light spectrum.
The first demonstration of the potential benefit of fluorescence imaging during surgery was provided in 2011. In that study, researchers from the University of Groningen’s BioOptical Imaging Center in Groningen (The Netherlands) showed that the use of tumor-specific fluorescence imaging offered specific and sensitive real-time identification of tumor tissue during surgery in ovarian cancer patients. The authors showed that seven times more malignant lesions could be detected in the belly by fluorescence imaging when compared with visual observation alone.
Subsequent research found that fluorescent dyes that emit in the NIR spectrum can enable the identification of deeper tumors, as Dr. Eben Rosenthal and his Dutch-American team reported in the January 2015 British Journal of Surgery.
Support us by buying our book, The Medicine of Light, and ebooks from our Photoimmune Discoveries eBook Series.
Sources
de Boer E, Harlaar NJ, Taruttis A, Nagengast WB, Rosenthal EL, Ntziachristos V, van Dam GM. Optical innovations in surgery. Br J Surg. 2015 Jan;102(2):e56-72.
Marescaux J, Diana M. Next step in minimally invasive surgery: hybrid image-guided surgery. J Pediatr Surg. 2015 Jan;50(1):30-36.
Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013 Sep;10(9):507-18.
Rosenthal EL, Warram JM, Bland KI, Zinn KR. The status of contemporary image-guided modalities in oncologic surgery. Ann Surg. 2015 Jan;261(1):46-55.
Handgraaf HJ, Verbeek FP, Tummers QR, Boogerd LS, van de Velde CJ1, Vahrmeijer AL, Gaarenstroom KN. Real-time near-infrared fluorescence guided surgery in gynecologic oncology: a review of the current state of the art. Gynecol Oncol. 2014 Dec;135(3):606-13. Valdes PA, Bekelis K, Harris BT, Wilson BC, Leblond F, Kim A, Simmons NE, Erkmen K, Paulsen KD, Roberts DW. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurgery. 2014;10 Suppl 1:74-82 © Copyright 2015, Photoimmune Discoveries, BV


 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands