Malignant Melanoma: A Better, Brighter Future for Treatment
If you’re like most people who think about the genesis of cancer, you’ve probably asked yourself why the immune system doesn’t seem to play a larger role in preventing and reversing this disease. After all, this system is supposed to provide immunity or protection against all manner of maladies, so why does it fail to do so in the case of cancer?
A number of answers have been provided for this perennial question. One is that cancer cells arise from normal tissues and lack certain features that would elicit an immune response—that is, they are have very little immunogenicity. In addition, most cancers evolve slowly over decades, allowing the disease to develop mechanisms for evading the immune system. Finally, conventional treatments such as chemotherapy and radiotherapy tend to suppress the immune sytem.
Unlike most cancers, however, malignant melanoma is a very immune-responsive or immunogenic disease. In other words, the immune system can determine whether malignant melanoma truly becomes a malignant threat in the first place, and moreover plays a major role in helping to eliminate this particular form of cancer.
Today’s medical scientists agree that more emphasis should be placed on harnessing and modulating the anti-cancer immune defenses to treat malignant melanoma. For advanced cases, a high-dose immunotherapy approach is often recommended, and this typically includes interferon and interleukin-2. Nonetheless, overall survival rates have not improved with the combined use of interferon and IL-2.
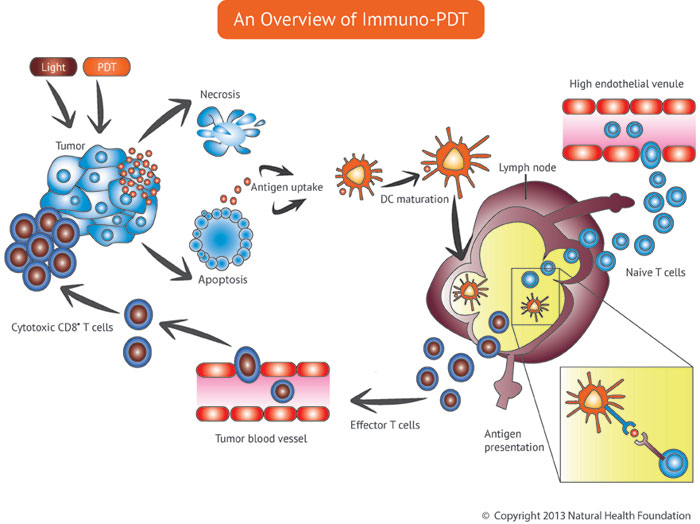
Other immunotherapy approaches are now being investigated, specifically the use of tumor vaccines to generate antibodies against the cancer. In addition, immune-based photodynamic treatments such as Immuno-PDT may prove useful.
Shining New Light on Immunotherapy
Immunotherapy entails the use of treatment strategies that harness the immune system against disease. This approach has become a mainstay treatment for malignant melanoma. At this time, however, most oncologists rely on a relatively small number of immune-enhancing strategies, and the results of these treatments for the more advanced-stage melanomas have been somewhat disappointing.
In the United States, for example, interleukin-2 and interferon-alfa 2b are the only approved immunotherapeutic drugs for melanoma treatment. These agents can result in remissions that sometimes last for years, but for many advanced-stage melanoma cases the chances of survival are poor. Moreover, the high cost and severe side effects of mainstream immunotherapy strategies have helped fuel an intense search for less toxic treatment options that might be used on a stand-alone basis or perhaps in combination with tumor vaccines and chemotherapy.
Recognizing the central role of the immune system for the control of some cancers, scientists have begun investigating the use of Photoimmunotherapy, or PIT, as a new therapeutic approach to malignant melanoma. In the patented approach to PIT developed by Andrei Reshetnickov, PhD, the photosensitizer is delivered through an infusion and treated with light as it is being infused into the body. This is very different from the usual strategy of photodynamic therapy (PDT), in which the photosensitizer is activated only after it has entered the target tissue.
With the PIT approach developed by Dr. Reshetnickov, the photosensitizer is already activated before it becomes concentrated in the melanoma cells, causing specific light-induced changes in their membranes (photomodification) and eventual destruction through light treatment. PIT could offer a very effective way to treat malignant melanoma, and research is under way using
Melanomas in the Eye
Though melanoma predominantly occurs in the skin, it can also occur in the iris and retina of the eye. The iris, ciliary body, or choroid are collectively referred to as the uvea, and thus melanomas occurring in these structures are general called uveal melanoma.
Tumors tend to arise from the pigment cells found within the uvea, giving the eye its particular color. Uveal melanoma is the most frequent intraocular cancer and the second most common form of melanoma. In half of patients with this type of melanoma, the cancer metastasizes and the prognosis is poor.
In a case series report published in the 23 January 2014 British Journal of Ophthalmology, 18 patients with posterior uveal melanoma were treated with a minimum of three sessions of PDT. In 83% of the cases, the tumor regressed with stable or improved vision over an average follow-up period of 28 months. None of the patients developed metastatic disease in the study period.
Thus, it appears that posterior uveal melanomas may be successfully treated with high-dose PDT, allowing for the retention of good vision in the majority of cases. However, a longer follow-up period is needed to see if these encouraging results are maintained.
More than 90% of uveal melanomas involve the choroid, and the remainder are confined to the ciliary body and iris. This makes choroidal melanoma the most common malignant tumor occurring inside the eye, and the second most common type of primary malignant melanoma in the body.
Recent clinical reports suggest that the PDT approach can lead to the regression of choroidal melanoma, as well as skin melanoma metastases. The anti-tumor effects of PDT result not only from the activation of an immune response, but also from the destruction of the tumor’s blood vessel supply and light-induced damage to the cancer cells themselves (thus causing a direct tumor-killing effect).
A Novel Photoimmune Approach: Adoptive Cell Transfer Plus PDT
Among the more innovative immunotherapy strategies is adoptive cell transfer, an approach that harnesses the T cell’s abiliity to attack cancer cells. T cells (also called T lymphocytes) are immune cells that can specifically target and destroy the patient’s cancer. These and other immune cells possess a unique homing capability that enables them to migrate to sites of inflammation initiated by injury, infection or cancer.
Some T cells can be found within the patient’s own tumor. These cells can be extracted from the tumor, then cultured in the laboratory before being transferred back into the patient with malignant melanoma. Genetically engineered T cells may also be used for this purpose, because not all cancers generate the tumor-specific T cells.
With the help of interleukin-2 (an immune-stimulating substance), the re-introduced T cells are essentially trained to attack the melanoma cells. One study found that this adoptive transfer strategy was an effective against metastatic melanoma, resulting in a 51% objective response, with long-lasting responses in 22% of the melanoma patients. In this study, however, only about one in every three patients survived for five years.
To improve the odds of survival, scientists have begun studying another option for guiding T cells to the cancer: the use of bispecific antibodies, engineered molecules that are able to recognize the cancer while also binding to part of the T cell. These antibodies can help guide the T cells to the cancer and may result in a stronger treatment effect.
Even more impressively, these same antibody-guided T cells can serve as a living drug delivery system to transport photosensitizing agents directly to the cancer. A team of researchers from the German Cancer Research Center and National Center for Tumor Diseases in Heidelberg and the Korea Institute of Science and Technology recently tried combining a porphyrin-based photosensitizer with this adoptive T-cell strategy.
In their initial studies, the scientists were able to show that the photosensitizer was internalized and tolerated by human T cells. More recently, they showed that the cancer-killing function of T cells was not impaired by the addition of the photosensitizer. Indeed, the photosensitizer-loaded T cells were more efficient in killing tumor cells than the antibody-guided but “unloaded” T cells.
The study’s main finding was that the combined tumor-killing potential of the photosensitizer-loaded T cells resulted in a synergy between the photodynamic effects and the immune-attack effects of the T cells. These findings will hopefully encourage the conduct of clinical studies of this novel T cell-based targeted PDT approach to malignant melanoma, as reported in the 10 January 2015 Journal of Controlled Release.
Support us by buying our book, The Medicine of Light, and ebooks from our Photoimmune Discoveries eBook Series.
Sources
Wang S, Riedinger A, Li H, Fu C, Liu H, Li L, Liu T, Tan L, Barthel MJ, Pugliese G, De Donato F, Scotto D’Abbusco M, Meng X, Manna L, Meng H, Pellegrino T. Plasmonic Copper Sulfide Nanocrystals Exhibiting Near-Infrared Photothermal and Photodynamic Therapeutic Effects. ACS Nano. 2015 Jan 30. [Epub ahead of print]
Murakami LS, Ferreira LP, Santos JS, da Silva RS, Nomizo A, Kuz’min VA, Borissevitch IE. Photocytotoxicity of a cyanine dye with two chromophores toward melanoma and normal cells. Biochim Biophys Acta. 2014 Dec 12. [Epub ahead of print]
Ogbodu RO, Ndhundhuma I, Karsten A, Nyokong T. Photodynamic therapy effect of zinc monoamino phthalocyanine-folic acid conjugate adsorbed on single walled carbon nanotubes on melanoma cells. Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 25;137:1120-5.
Rundle P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol. 2014 Jan 23. [Epub ahead of print] Rundle P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol. 2014 Jan 23. [Epub ahead of print]
Blaudszun AR, Moldenhauer G, Schneider M, Philippi A. A photosensitizer delivered by bispecific antibody redirected T lymphocytes enhances cytotoxicity against EpCAM-expressing carcinoma cells upon light irradiation. J Control Release. 2015 Jan 10;197:58-68.
Rundle P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol. 2014 Apr;98(4):494-7.
© Copyright 2015, Photoimmune Discoveries, BV

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands